Blog Post
Smoking Cessation in the Workplace: What Works?
Three Takeaways from NEJM Study
My colleagues and I, including Kevin Volpp and Michael Harhay, recently published results of a large smoking cessation trial in the New England Journal of Medicine. We tested the real-world effectiveness of offering five strategies for quitting smoking among all known smokers at 54 U.S. companies (over 6,000 employees), regardless of their interest in quitting. The results reflect what employers, insurers, or others offering similar programs might expect to see. I want to share some thoughts on the trial, including what we can and cannot conclude, and answer some questions I’ve heard so far.

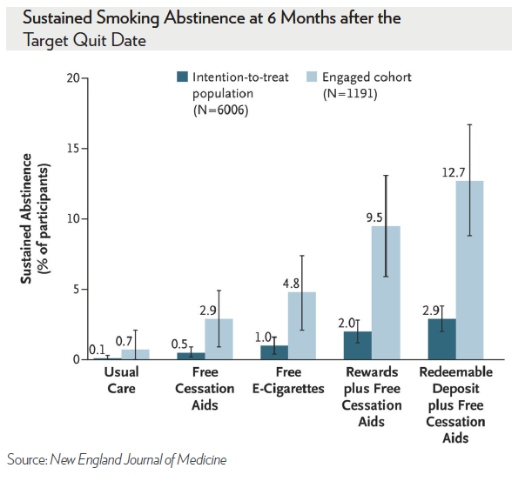
We found that $600 – offered as a pure carrot, or a “soft stick” – when added to free nicotine replacement therapy and U.S. Food and Drug Administration (FDA)-approved drugs, tripled quit rates compared to offering free smoking cessation aids alone. Financial incentives were also more effective at improving quit rates than electronic cigarettes (e-cigarettes).
In fact, neither free cessation aids nor e-cigarettes improved quit rates among employees who were offered information about the benefits of quitting. Contrary to expectations from our prior work, the “soft stick,” which made people feel the money was theirs to lose, was not better than framing the financial incentive as a reward.
Importantly, these findings of the relative effectiveness of the strategies were identical among all smokers and among the 20 percent of smokers who actively engaged with the study. The engaged employees were better educated and more motivated to quit. As expected, this latter group had much higher absolute quit rates.
There’s been understandable confusion about how we measured who quit. To be considered “quit,” smokers had to submit three urine or blood samples over six months at a Quest Diagnostics lab showing no chemicals unique to regular cigarettes. Those still using e-cigarettes or nicotine replacement therapy were considered to have quit.
There have also been questions about whether people offered free smoking cessation aids and e-cigarettes actually used them. For reasons of space, we had to put these data in the online supplement (Table S11). But in short, yes, many people offered free aids or e-cigarettes used them.
Finally, some have wondered how we could have found that incentive programs were so much more cost-effective than offering free cessation aids or e-cigarettes. There are two reasons: they were more effective at getting employees to stop smoking, and costs only accrue for those who quit. The costs of aids accrue for anyone who uses them.
My take-homes: As always, more research is needed, including on different types of e-cigarettes and particularly among underserved populations (i.e., not employees) in whom the burdens of smoking are greatest. But we can still say several things with confidence:
First, it is now clear from three large randomized controlled trials that incentives triple quit rates regardless of whether we look at all-comers or just those motivated to quit, and regardless of what other therapies are offered in the background.
Second, in this trial, the largest and most robust trial to date, e-cigarettes did not help people quit smoking. This is important because there is no compelling evidence that they do promote smoking cessation, as noted in a recent report by the National Academies of Sciences, Engineering, and Medicine, and as the FDA considers regulating e-cigarettes.
Third, employers, insurers, health systems and others who are commonly offering free nicotine replacement therapy and smoking cessation drugs might do better for the health of those they serve and their own financial bottom lines by offering incentives instead of or in addition to those aids.
[This content originally appeared as a thread of tweets by Scott Halpern. Follow him @ScottHalpernMD.]