Methadone Use Rises—But Too Few People Get Opioid Medication
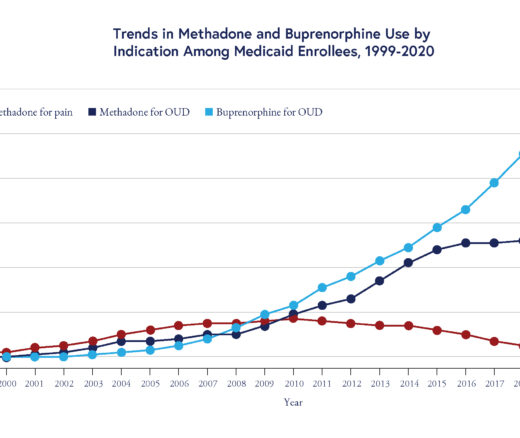
Chart of the Day: Methadone Use for Opioid Use Disorder Tripled From 2010–2020, Yet Only One in Four People With Addiction Receive Medication
Substance Use Disorder
Blog Post

When someone is prescribed opioids, evidence shows that co-prescribing or offering naloxone to quickly reverse overdoses is associated with fewer deaths. However, even though the U.S. Food and Drug Administration (FDA) has approved it for children, policy and practice has rarely reflected the specific needs of children and families. This puts children at avoidable risk.
LDI Fellow Barbara H. Chaiyachati and colleagues believe it is time to more deeply consider the unique needs of children and families for naloxone access. Across the country, requirements vary for discussing, offering, or prescribing this overdose-reversal drug. Many regulations primarily focus on prescriber behavior, though some states allow pharmacy-level intervention. Even in the 18 states with mandates in 2023 for co-prescribing naloxone with opioids, legislation does not always consider children. For example, the required opioid dose for naloxone co-prescription can range from more than 50 morphine milligram equivalents (MME) per day to over 120 MME per day. The full range is well above the level of toxicity for many small children.
The researchers have published guidelines for parents and recommend that health care providers, institutions, and policymakers consider the following actions to keep children safe from overdose.
Proactively discuss medication safety and opioid reversal with patients and let them know that naloxone is available over the counter.
When prescribing opioids, discuss naloxone and how to use it.
Standardize how to discuss, offer, or prescribe naloxone in qualifying patients including children and adults who are frequently around children.
Consider including naloxone in professional society opioid prescribing guidelines such as reflected in the 2024 American Academy of Pediatrics (AAP) opioid prescribing clinical practice guideline.
In regulations, consider removing dosing ranges or other qualifying circumstances or uncoupling them from risk criteria in children.
Consider including more family structures in legislation, such as those consistent with 2022 CDC guidance.
The viewpoint “Consideration of Children in Naloxone Coprescribing Laws” was published on November 24, 2024 in JAMA Pediatrics. Authors include Ava Hunt, Jeanette Trella, and Barbara H. Chaiyachati.


Chart of the Day: Methadone Use for Opioid Use Disorder Tripled From 2010–2020, Yet Only One in Four People With Addiction Receive Medication

LDI Fellows Used Medicaid Data to Identify Individuals at Highest Risk for Cocaine- and Methamphetamine-Related Overdoses, Paving the Way for Targeted Prevention

Penn and Four Other Partners Focus on the Health Economics of Substance Use Disorder

Penn Medicine’s New Summer Intern Program Immersed Teens in Street Outreach Techniques

LDI Experts Offer 10 Solutions to Get More Help to Seniors With Addiction

More Flexible Methadone Take-Home Policy Improved Patient Autonomy