How to Lower Maternal and Child Deaths Across the World? Give Low-Income Families Cash.
Research Brief: Cash Transfer Programs Improve Birth, Nutrition, and Early Childhood Health Outcomes
Population Health
Blog Post
Intended to offset cuts in federal Medicaid funding and empower states to strengthen rural health care, the Rural Health Transformation Program (RHTP) was created in July 2025 through the One Big Beautiful Bill Act. From 2026 to 2030, the RHTP allocates $10 billion each year- distributing $5 billion worth of ‘baseline funding’ equally, with each state receiving $100 million annually, and the remaining $5 billion in ‘workload funding’ based on state characteristics and proposals.
After the first year of RHTP funding amounts were announced in December 2025, LDI Director of Health Equity Research Paula Chatterjee, LDI Executive Director Rachel M. Werner, and LDI Statistical Analyst Eliza Macneal conducted a cross-sectional analysis to see whether the allocations reflected states’ rural health needs.
States like Texas and North Carolina, with larger rural populations, received less RHTP funding per resident. The figure below shows both total and per-rural-resident RHTP funding amounts, and orders states from highest to lowest rural population. Researchers found no clear connection between how many rural residents a state had and how much funding it received.
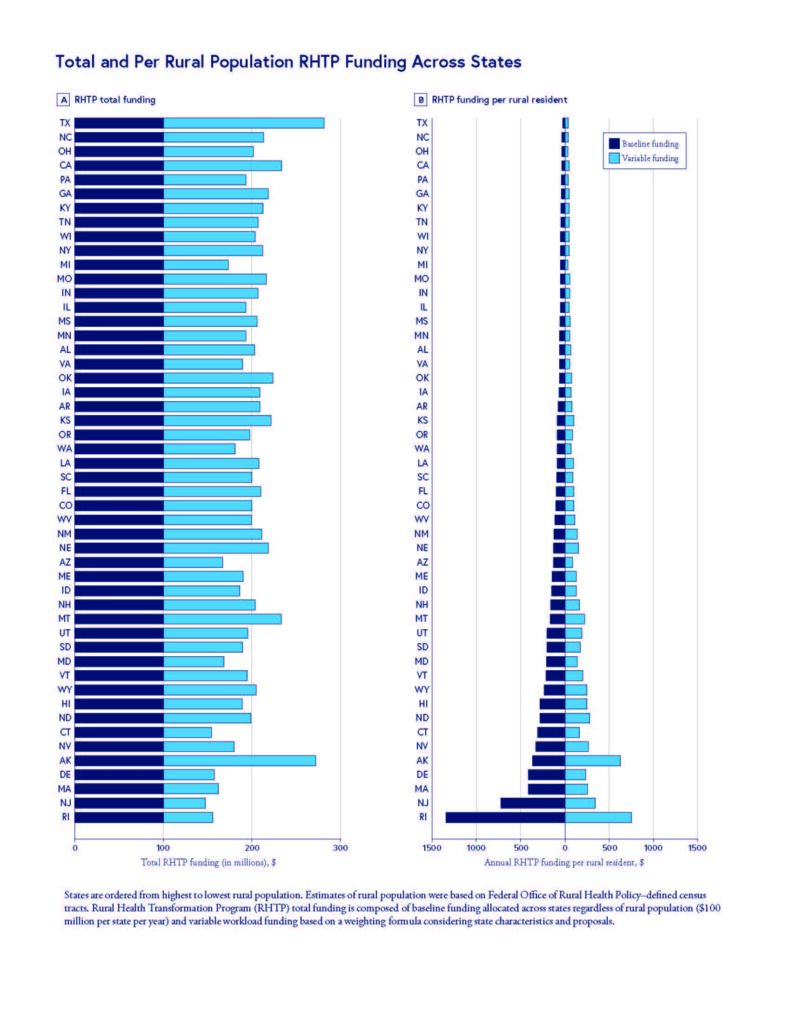
The team also found that states with the lowest rural death rates – Hawaii, Massachusetts, Colorado – received more RHTP funding per-rural-resident, while states with the highest death rates –Mississippi, Kentucky, Tennessee – received less. This inverse correlation between rural mortality and per-rural-resident funding raises concerns about whether RHTP funds may reach rural residents with greatest clinical need.
Based on projected 10-year federal Medicaid spending reductions under the One Big Beautiful Bill Act from the Kaiser Family Foundation, the team found that states projected to have smaller Medicaid cuts received more RHTP funding per rural resident while those predicted to have larger Medicaid cuts received less.
In addition, states that lost rural hospital beds from 2017 to 2022 received less RHTP funding per rural resident compared to states that gained beds. There was also little correlation between RHTP funding per rural resident and whether a state lost or gained rural physicians per rural resident from 2017 to 2022.
“Mortality, hospital bed loss, and physician workforce changes are imperfect proxies for need, and many rural areas lost rural hospitals and physicians prior to 2017,” Chatterjee said. However, the findings indicate that the December 2025 allocations of RHTP funding may not align with where rural health and access challenges may be greatest.
“Without mechanisms to explicitly target clinical need, allocation strategies may potentially exacerbate existing rural health disparities — the opposite of RHTP’s goals,” Werner said. The team suggests that policymakers consider more closely aligning future RHTP funding allocations with states’ rural health needs to better reach the program’s goals.
The article, “Rural Health Transformation Program Allocations and Rural Health Needs in the US,” was published in JAMA on March 5, 2026. Authors include Paula Chatterjee, Eliza Macneal, and Rachel M. Werner.

Research Brief: Cash Transfer Programs Improve Birth, Nutrition, and Early Childhood Health Outcomes

Without Pressure From Congress, NHANES — Which Helped Uncover High Levels of Childhood Lead, Nutritional Deficiencies, and Forever Chemicals — Will Cease to Exist

A Multi-State Study Finds That Parents Often Travel 60+ Miles—With Distance, Insurance, and Race Driving Gaps in Maternal Care

Cheaper Housing Could be a Way to Lower Hospitalizations Among Medicaid Patients with Heart Failure

Pa.’s New Bipartisan Tax Credit is Designed to be Simple and Refundable – Reflecting Core Points From Penn LDI Researchers Who Briefed State Leaders

Announcing Bold New Goals While Crippling the Infrastructure Needed to Achieve Them