
Only One in Four High-Risk Rural Births Get Appropriate Hospital Care
A Multi-State Study Finds That Parents Often Travel 60+ Miles—With Distance, Insurance, and Race Driving Gaps in Maternal Care
Blog Post
The Inflation Reduction Act of 2022 allows Medicare, for the first time, to negotiate the price of prescription drugs. The new law tackles a major health policy goal to make health care—and pharmaceuticals in particular—more affordable. However, long before 2022, Congress created an obscure but influential pharmaceutical program intended to shore up safety net institutions and improve access to care: the 340B Program.
The 340B Program, created in 1992, began as a way to financially strengthen safety-net health care institutions without the government having to pay. It required that pharmaceutical companies provide steep drug discounts to eligible safety net hospitals or clinics, the recipients would then be able to earn larger margins when receiving standard reimbursement from insurers. The funds that these 340B providers preserved through this arrangement could then, ideally, be passed along to patients directly through access to lower-cost drugs or indirectly through charity care and community investments.
In 2010, in response to continued concerns about lack of access to medications in disadvantaged communities—“pharmacy deserts”—the 340B program was expanded so that safety net institutions could contract with an unlimited number of pharmacies, thereby expanding the distribution of discounted drugs. When patients fill discounted drugs from these participating pharmacies, the 340B providers and contract pharmacies share the surplus.
The intention was to encourage pharmacies to stay open in disadvantaged communities and enable the pharmacies to pass the drug discounts on to low-income patients. But what came next shows how policies with good intentions can go awry.
Our research, funded by the LDI Small Grants Program and published recently in JAMA Health Forum, generated evidence that the 340B discounted drugs appear to go more and more to pharmacies in wealthier communities.
Between 2006 and 2019, the percentage of 340B pharmacies declined by 5.6% in the lowest income neighborhoods and by 3.2% in Black neighborhoods. In contrast, the percentage of 340B pharmacies increased by 5.0% in the highest income neighborhoods and by 4.0% in predominantly White neighborhoods. This shift occurred even though the share of all retail pharmacies in socioeconomically disadvantaged and minoritized neighborhoods increased slightly. Furthermore, large retail pharmacy companies have benefited as the majority of the pharmacies contracting in the 340B Program have been massive chains such as CVS and Walgreens.
Large urban hospital systems have also profited from the 340B Program. The participation requirements of 340B are calculated based on a hospital system’s inpatient population. These hospital systems can remain in the 340B Program even when they augment their outpatient case mix with privately insured and Medicare patients, so long as this practice only minimally impacts their inpatient case mix.
Furthermore, hospitals participating in the 340B Program have found it increasingly more rewarding as more costly drugs have entered the market. Researchers—including those in our team—have found that hospital systems that entered the program more recently increased their profits more than hospital systems that entered the program earlier. Profits came from expanding outpatient facilities to wealthy neighborhoods, buying up lucrative oncology practices, and charging private insurers marked-up prices for expensive infusion drugs. This all occurred without evidence that the profits gained through their participation in the 340B program were going to their most vulnerable patients (here, here, and here).
A U.S. Government Accountability Office report found the majority of hospitals chose not to share 340B discounts with their patients even though these discounts could help to cover the rising copays for expensive specialty medicines. The choice of many 340B hospitals not to share discounts with their patients has occurred despite evidence that patients struggle to pay for their prescriptions: Recent research found that 30% of Medicare cancer beneficiaries leave their oral anticancer prescriptions unfilled.
Controversy surrounding the 340B Program has intensified, with some of the largest organizations in health care jockeying to financially strengthen their position within the program. In November 2017, the Centers for Medicare and Medicaid Services (CMS) finalized a rule to slash reimbursements to 340B hospitals, but the cut was reversed by the Supreme Court.
Meanwhile, 18 pharmaceutical companies unilaterally restricted 340B discounts to their products, an act deemed illegal by the Health Resources and Services Administration. Seven of these companies have been referred to the Department of Health and Human Services Office of Inspector General to assess whether the manufacturers should face penalties for “knowingly and intentionally” overcharging for 340B drugs.
Such actions may curb the excesses of larger wealthy hospital systems; unfortunately, they also damage smaller safety net institutions. More than 130 rural hospitals closed over the past 10 years, with the number of closures hitting a high in 2020. In a survey sponsored by an advocacy group called 340B Health, rural hospitals reported that pharmaceutical company restrictions led to losses of $500,000 to $1 million per year. Blunt cuts to the 340B Program could make it even harder for struggling hospitals to stay open. Indeed, other researchers recently found, in contrast with 340B pharmacy contracts with hospitals, pharmacy contracts with safety net clinics have grown in areas with higher poverty rates.
The 340B Program has great potential to help the most vulnerable Americans, but it is in need of reform. There is no shortage of opinions for how to do so, ranging from restricting the types of institutions that can be eligible to providing more explicit regulations on who gets to use the money and how—for instance by requiring that pharmacies and providers pass along some of the discounts to low-income patients struggling with co-pays. The tricky part will be getting any potential reform right so that the benefits are targeted to patients and institutions that need it most.
The research letter, “Assessment of U.S. Pharmacies Contracted With Health Care Institutions Under the 340B Drug Pricing Program by Neighborhood Socioeconomic Characteristics,” was published in the June 17, 2022, issue of JAMA Health Forum. Authors include John K. Lin, Pengxiang Li, Jalpa A. Doshi, and Sunita M. Desai.





A Multi-State Study Finds That Parents Often Travel 60+ Miles—With Distance, Insurance, and Race Driving Gaps in Maternal Care

Former CMMI Leader Liz Fowler Cites Rigid Federal Scoring Rules and Bureaucratic Impatience for Pilot Failures
A Major European–U.S. Hospital Study Finds That Changing How Hospitals Are Organized Reduces Burnout and Turnover While Improving Care Quality

Penn LDI Senior Fellow Dominic Sisti Cites “Alarming Levels”
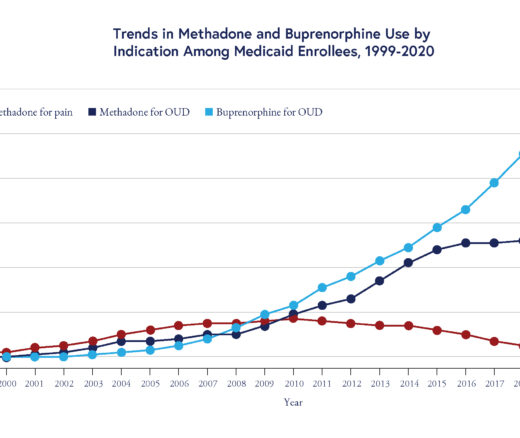
Chart of the Day: Methadone Use for Opioid Use Disorder Tripled From 2010–2020, Yet Only One in Four People With Addiction Receive Medication
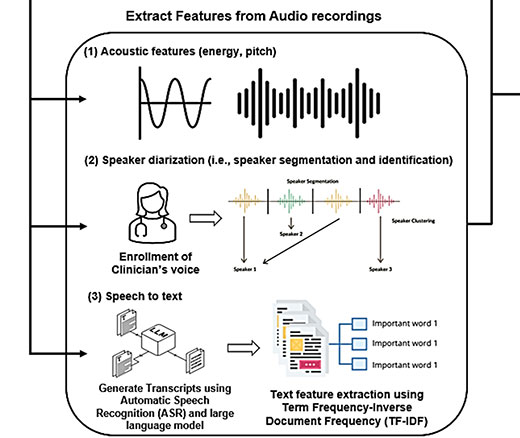
Researchers Use AI to Analyze Patient Phone Calls for Vocal Cues Predicting Palliative Care Acceptance