
Acupuncture Could Fix America’s Chronic Pain Crisis–So Why Can’t Patients Get It?
A Proven, Low-Risk Treatment Is Backed by Major Studies and Patient Demand, Yet Medicare and Insurers Still Make It Hard To Use
Blog Post
The Accelerated Approval (AA) Program serves a key role by getting promising drugs to patients faster. The program, created during the HIV/AIDS crisis in 1992 by the Food and Drug Administration (FDA), focuses on diseases with unmet needs and approves drugs based on surrogate end points—a shrinking tumor or lower biomarkers, for example—that can stand in for overall survival. And while that process leads to faster access, it raises concerns that we are exposing patients to treatments that are later shown to provide no benefit.
Indeed, the program has seen a rise in withdrawals of indications for these medicines, which are mostly in oncology. From 2009 to 2022, the AA program approved 48 drugs and 66 oncology-related indications. But 15 indications (23%) were withdrawn due to lack of benefit. In a research letter published last month in JAMA Oncology, LDI Senior Fellow Ravi B. Parikh and his team at the University of Pennsylvania estimated how many patients were receiving these ineffective treatments.
Parikh, an oncologist, researcher, and Director of the Human Algorithm Collaboration Lab (HACLab) which focuses on advanced algorithms in clinical care and health policy, spoke more about the study.
For the first time, our study has shown what percentage of patients are affected when indications are withdrawn for these oncology drugs. We studied five different indications for breast, bladder, liver, gastric, and small cell lung cancers using a large real-world dataset that contains granular information on oncology indications. That’s important because most datasets just have information on what type of cancer patients have and what drug was used. Because of that, you cannot get a good denominator or total population of patients who received certain lines of therapies or with certain biomarkers, which is necessary to calculate the percentage of patients affected by accelerated approval drugs.
We selected several indications that are commonly used, and learned what percent of the time an eligible patient actually received an accelerated approval drug.
Across each of the five indications, about one-fourth (26%) of eligible patients got a drug that was subsequently withdrawn. In some cancer indications, like one in small cell lung cancer, that number was 41%. These drugs always had negative trials published; after approval, but before the negative trial was published, about one-third of eligible patients received an accelerated approval drug that was subsequently withdrawn. What this shows is that the use of withdrawn accelerated approval drugs is really affecting a large population. That presses the FDA to figure out this problem sooner by either 1) generating confirmatory evidence faster so that fewer patients are exposed to ineffective drugs, or 2) ensuring adequate standards for the initial trials so that ineffective drugs don’t make it to market in the first place.
No. A lot of people on Twitter want to take it down, but my co-workers and I are not in that camp. I have personally seen many patients who have had earlier access to accelerated approval drugs and benefit from them, before their standard phase 3 trial is completed.
Most accelerated approval drugs work out. Only 20-25% of drugs are withdrawn, which is a good success rate. The critics are not considering the 70-75% of drugs that are working.
For drugs that show signs of being ineffective in larger scale trials, we need to get the negative information on these drugs out faster. Right now, it takes 46 months after accelerated approval (and for one drug, 58 months) for an ultimately ineffective drug to be withdrawn by the FDA.
The Food and Drug Omnibus Reform Act of 2022 (FDORA), part of the Consolidated Appropriations Act, that passed in late December, enables the FDA to require the launch of a confirmatory trial before accelerated approval is granted. A phase 3 trial should already be enrolling patients when a drug gets accelerated approval. That would decrease the 46 months of exposure to drugs that don’t work. FDORA also gives the FDA the option of using faster procedures to withdraw approval.
Some additional things are needed. In phase 2 trials, we ought to be using higher standards for approval than surrogate markers; one of those markers could include overall survival. Right now, accelerated approval is usually based on phase 2 studies that use surrogate end points as a proxy for overall survival. We need to use harder end points and compare the new work to historical controls. Or we need to get larger numbers of patients enrolled.
We are interested in studying the effectiveness of newer generations of accelerated approval drugs in the oncology setting. We are also really interested in studying outcomes other than clinical effectiveness, things like adverse event rates and cost, that may better contextualize the costs and benefits of these drugs. Finally, given the recent FDA legislation, we really want to evaluate its effectiveness in future observational studies.
The study, “Exposure to U.S. Cancer Drugs With Lack of Confirmed Benefit After U.S. Food and Drug Administration Accelerated Approval” was published on February 23, 2023 in JAMA Oncology. Authors include Ravi B. Parikh, Rebecca A. Hubbard, Erkuan Wang, Trevor J. Royce, Aaron B. Cohen, Amy S. Clark, and Ronac Mamtani.


A Proven, Low-Risk Treatment Is Backed by Major Studies and Patient Demand, Yet Medicare and Insurers Still Make It Hard To Use
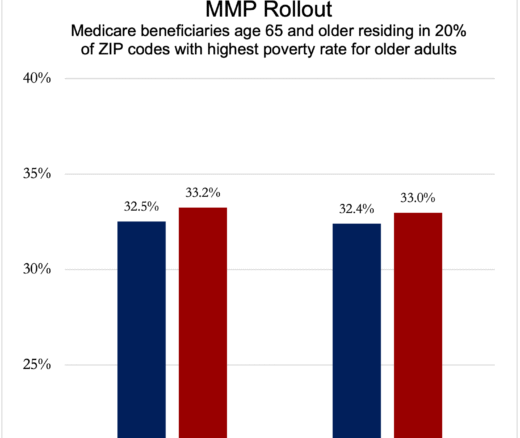
Chart of the Day: Medicare-Medicaid Plans—Created to Streamline Care for Dually Eligible Individuals—Failed to Increase Medicaid Participation in High-Poverty Communities
Research Brief: Shorter Stays in Skilled Nursing Facilities and Less Home Health Didn’t Lead to Worse Outcomes, Pointing to Opportunities for Traditional Medicare

How Threatened Reproductive Rights Pushed More Pennsylvanians Toward Sterilization

Abortion Restrictions Can Backfire, Pushing Families to End Pregnancies

They Reduce Coverage, Not Costs, History Shows. Smarter Incentives Would Encourage the Private Sector