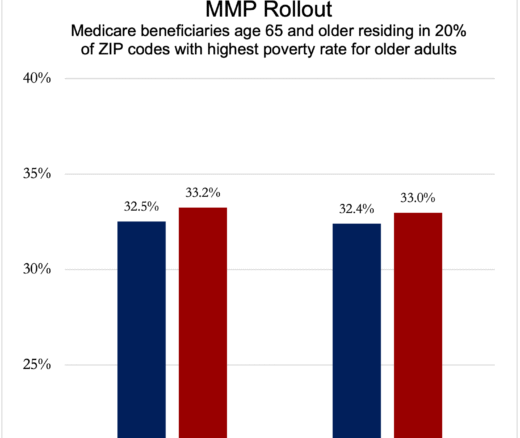
Integrated Care Plans Didn’t Boost Medicaid Enrollment for the Poorest Seniors
Chart of the Day: Medicare-Medicaid Plans—Created to Streamline Care for Dually Eligible Individuals—Failed to Increase Medicaid Participation in High-Poverty Communities
Blog Post

Taking a health-related blood test at home is convenient for patients. It is also quite lucrative for companies producing direct-to-consumer (DTC) tests. The market is expected to be worth $2 billion by 2025 and is predicted to have an annual growth rate of over 18%.
Yet despite enabling broad access to sensitive health data and widespread use among consumers, little is known about protections for patients who use the tests.
A recent research letter in JAMA Network Internal Medicine by LDI Senior Fellow Anna Wexler and her former postdoctoral fellow Louiza Kalokairinou, now of Baylor College of Medicine, explored DTC home health testing practices through online exploration of company websites. They examined companies’ terms of service (ToS) and privacy policies, and evaluated their protocols for abnormal result delivery and health care professional involvement.
Among 21 companies evaluated, 18 different DTC tests were included in the website sample. The companies offered a range of tests for chronic conditions such as diabetes and thyroid disease. Most required the collection of blood either through at-home finger sticks or blood draws performed at walk-in laboratories. Some collected other fluids via swab or another sampling procedure.
One key finding is that the terms of these tests shift responsibility to the consumer to determine the results’ accuracy and usefulness. While consumers may expect privacy protections similar to seeing their doctors, DTC firms don’t offer that level of security. The only protections come from the fine print consumers agree to when they access testing.
Company websites post disclaimers about the test results not constituting medical advice, and limitations of liability were common. Many sites asserted that their service was provided to improve communication between individuals and health professionals. Risk information and declared compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) was minimal. Few companies specified privacy protections for biological samples.
Companies did report patient privacy protections regarding website data, though the strictness of these policies varied. Only four companies stated that consumers could request the deletion of their data, and more than half indicated the potential use of consumer data for research purposes.
We asked Wexler and Kalokairinou about the origin of their work and the implications of their research for patients and policymakers. Since companies stated that their products were designed to promote physician-patient communication, we also asked David Grande, a practicing internist who is also the LDI Director of Policy and an Associate Professor of Medicine at the Perelman School of Medicine, for his thoughts.
Wexler: The DTC home health testing market has been booming, and like other forms of DTC health care, it seems to have gained additional traction during the COVID-19 pandemic. Yet in my experience, surprisingly few physicians are aware that consumers can purchase lab tests directly. So it’s a relatively novel space, and there hasn’t been a tremendous amount of attention to ethics in this.
Kalokairinou: DTC testing has created a shift of products and services that have been traditionally offered in health care settings, into the consumer realm. While this shift cuts out the middleman and may empower consumers to make their own health care decisions, it also takes away some of the safeguards that tend to be associated with conventional health care, such as medical supervision and doctor-patient relationships, and creates a gray area between consumer and health care products.
Grande: I worry about DTC tests being marketed to consumers who may not benefit from the testing. We want innovation that improves access but we also don’t want consumers wasting their money on tests they do not need. In addition, we want to make sure information is communicated to their primary care provider so people can be treated appropriately for conditions like diabetes and thyroid disease. If patients take these tests and doctors never see the results, it will not improve their health.
Wexler: From my perspective, it was really the dissonance between the way DTC lab tests are marketed as empowering consumers to gain access to health and medical information, and the details of the ToS, which sometimes took great pains to acknowledge that information provided was for “informational” or “educational” purposes only. That, and the fact that fewer than half of the companies in our sample explicitly stated that they were HIPAA compliant.
Kalokairinou: What surprised me the most is the vagueness of many of these policies, especially regarding how personal data are protected, what happens to the samples of consumers, and under what conditions this information may subsequently be used for other purposes, such as research. Such vagueness in terms and conditions is not unusual in the case of consumer products. However, given the sensitivity of the data involved in DTC health testing, the risks to consumers may be greater in this case.
Wexler: They should know that in some cases it may be a cheaper alternative to testing they can obtain via a referral from a physician’s office–but that their health data may not be protected in the way they’d expect from other health services and products.
Kalokairinou: Patients should know that while these tests are easily accessible, convenient, and in some cases cheaper than tests offered in traditional health care settings, the terms and conditions of the companies shift responsibility to the consumer to evaluate the accuracy and usefulness of the information provided. In addition, consumers need to be cognizant of the sensitivity of the health information they provide companies and carefully evaluate the companies’ privacy policies in order to decide whether the benefits of the test outweigh the risks.
Grande: Consumers likely expect that their privacy would be protected just like when they visit their doctor. That is not true for DTC companies. The only protections come from the fine print consumers are asked to agree to when they access testing. It is impossible to read or comprehend unless you are a legal expert. Consumers assume that their information will be private but this study shows that protections are lacking.
Wexler: The Food and Drug Administration (FDA) is currently revisiting its policy for laboratory-developed tests, so it’s likely we’ll see some policy change in this space in the near future. There are many issues to consider here, including ensuring the accuracy and validity of test results; establishing protocols for the return of results and dealing with abnormal results; protecting the privacy of biological samples as well as health data; and increasing transparency by requiring that companies provide the names and contact information for ordering physicians.
Kalokairinou: There are many different aspects that policymakers need to consider when regulating this industry. Overall, policymakers need to strike a balance between promoting consumers’ autonomy by facilitating access to health care products and services, and protecting consumers from overly broad and vague policies that may not enable them to make informed decisions.
Grande: Patients need to know three things–that results are accurate, information will be kept private, and whether they will benefit from testing in the first place. Policymakers should consider strategies that can address all three–quality, privacy, and clinical value.
Wexler: In my lab we’ve conducted a series of studies on DTC health technology, ranging from policy issues related to DTC neurotechnology to perspectives of users of DTC orthodontic products to ethical issues in DTC COVID tests. We’ve been seeing scholars raise similar ethical questions about DTC health products across a variety of medical domains, so at the moment we’re completing a scoping review of ethical issues raised in the literature across different types of DTC products and treatments.
The study, “Policies of US Companies Offering Direct-to-Consumer Laboratory Tests,” was published on September 18, 2023 in JAMA Network Open Internal Medicine. Authors include Louiza Kalokairinou, Rebekah Choi, Nina (Yichen) Wei, and Anna Wexler.


Chart of the Day: Medicare-Medicaid Plans—Created to Streamline Care for Dually Eligible Individuals—Failed to Increase Medicaid Participation in High-Poverty Communities
Research Brief: Shorter Stays in Skilled Nursing Facilities and Less Home Health Didn’t Lead to Worse Outcomes, Pointing to Opportunities for Traditional Medicare

How Threatened Reproductive Rights Pushed More Pennsylvanians Toward Sterilization

Abortion Restrictions Can Backfire, Pushing Families to End Pregnancies

They Reduce Coverage, Not Costs, History Shows. Smarter Incentives Would Encourage the Private Sector
Research Brief: Less Than 1% of Clinical Practices Provide 80% of Outpatient Services for Dually Eligible Individuals